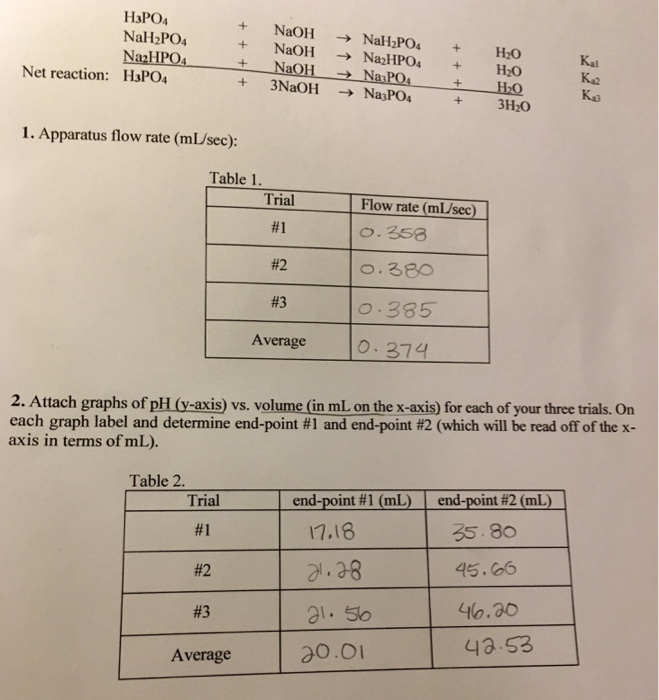
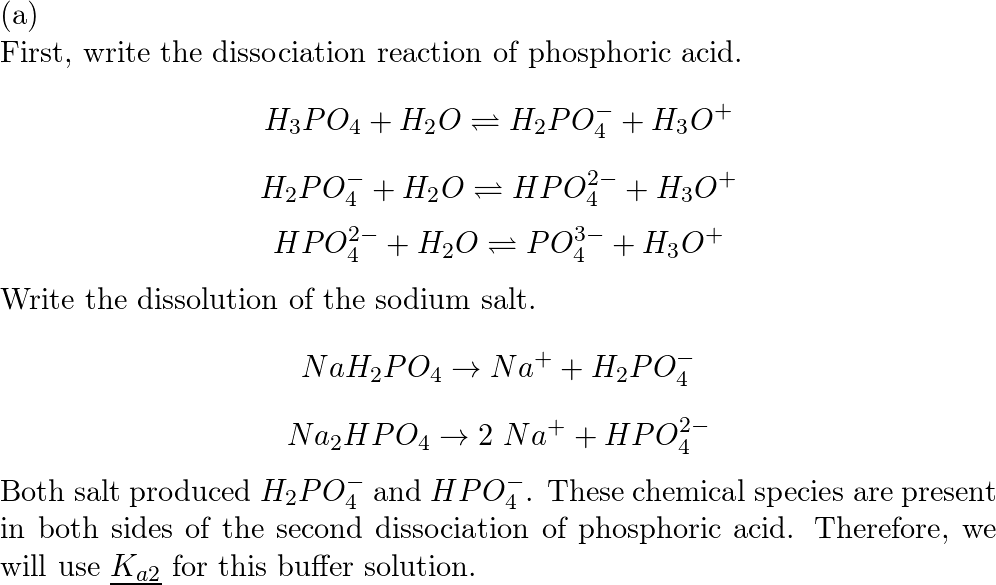1 Bán Hóa chất Sodium dihydrogen phosphate monohydrat, reagent grade - NaH2PO4.H2O - SO0331 - Scharlau giá rẻ ở hcm

PDF) Hydrogen Bonding in the Crystalline State. NaH2PO4, a Crystal Structure with a Short Asymmetrical Hydrogen Bond
![Sodium Phosphate Monobasic dihydrate (NaH2PO4.2H2O, 1kg) [CN04-1KG] - $36.00 : Bioland Scientific, for Your Research Needs Sodium Phosphate Monobasic dihydrate (NaH2PO4.2H2O, 1kg) [CN04-1KG] - $36.00 : Bioland Scientific, for Your Research Needs](https://www.bioland-sci.com/images/NaH2PO4s%201KG.jpg)
Sodium Phosphate Monobasic dihydrate (NaH2PO4.2H2O, 1kg) [CN04-1KG] - $36.00 : Bioland Scientific, for Your Research Needs
![Sodium Phosphate Monobasic Monohydrate [Sodium dihydrogen Phosphate monohydrate], 1 Kilogram: Amazon.com: Industrial & Scientific Sodium Phosphate Monobasic Monohydrate [Sodium dihydrogen Phosphate monohydrate], 1 Kilogram: Amazon.com: Industrial & Scientific](https://m.media-amazon.com/images/I/41zZzkwboAL._SR600%2C315_PIWhiteStrip%2CBottomLeft%2C0%2C35_SCLZZZZZZZ_FMpng_BG255%2C255%2C255.jpg)
Sodium Phosphate Monobasic Monohydrate [Sodium dihydrogen Phosphate monohydrate], 1 Kilogram: Amazon.com: Industrial & Scientific
![Sodium Phosphate Monobasic dihydrate (NaH2PO4.2H2O, 500g) [CN04-500G] - $20.00 : Bioland Scientific, for Your Research Needs Sodium Phosphate Monobasic dihydrate (NaH2PO4.2H2O, 500g) [CN04-500G] - $20.00 : Bioland Scientific, for Your Research Needs](https://www.bioland-sci.com/images/NaH2PO4s%20500G.jpg)
Sodium Phosphate Monobasic dihydrate (NaH2PO4.2H2O, 500g) [CN04-500G] - $20.00 : Bioland Scientific, for Your Research Needs
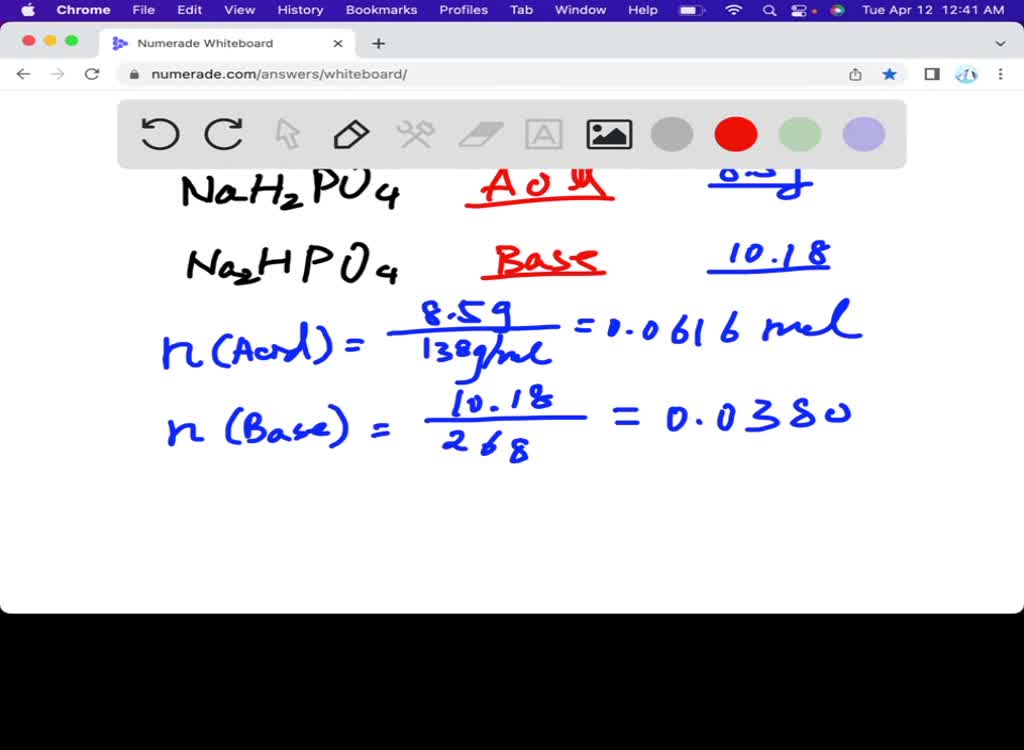
SOLVED: Calculate the mass of NaH2PO4 ∙ H2O (in grams) required to prepare 250mL of pH 7.21 buffer solution if the total buffer concentration is to be 0.20M. The Ka of NaH2PO4∙H2O

10049-21-5, 137.99, Sodium Phosphate, Monobasic, Monohydrate, Crystal, Reagent, ACS - 6NNX9|S1395-500GM - Grainger
![Sodium Phosphate Monobasic Monohydrate [Sodium dihydrogen Phosphate monohydrate], 1 Kilogram: Amazon.com: Industrial & Scientific Sodium Phosphate Monobasic Monohydrate [Sodium dihydrogen Phosphate monohydrate], 1 Kilogram: Amazon.com: Industrial & Scientific](https://m.media-amazon.com/images/I/71S3q3Er55L.jpg)
Sodium Phosphate Monobasic Monohydrate [Sodium dihydrogen Phosphate monohydrate], 1 Kilogram: Amazon.com: Industrial & Scientific

Phase Equilibrium for the Ternary System KH2PO4 + NaH2PO4 + H2O at 303.15 K | Journal of Chemical & Engineering Data

Phase Equilibrium for the Ternary System KH2PO4 + NaH2PO4 + H2O at 303.15 K | Journal of Chemical & Engineering Data

SOLVED: What is the balanced equation and the net ionic equation. NaH2PO4 + Na2HPO4 + H2O + MgCl2 ⇒ NaH2PO4 + Na2HPO4 + H2O + NaCl ⇒ ( we did an experiment

10049-21-5, 137.99, Sodium Phosphate, Monobasic, Monohydrate, Crystal, Reagent, ACS - 6NNX9|S1395-500GM - Grainger

Jual Sodium dihydrogen phosphate monohydrate/Nah2PO4.h2O - Kab. Bantul - muda berkah jogja | Tokopedia

Organic-NaH2PO4-H2O aqueous biphasic system for extraction of paeonol from cortex moutan: Solvent selection and mechanism probing - ScienceDirect

Phase Equilibrium for the Ternary System NaH2PO4 + Na2SO4 + H2O in Aqueous Solution at 298.15 K | Journal of Chemical & Engineering Data