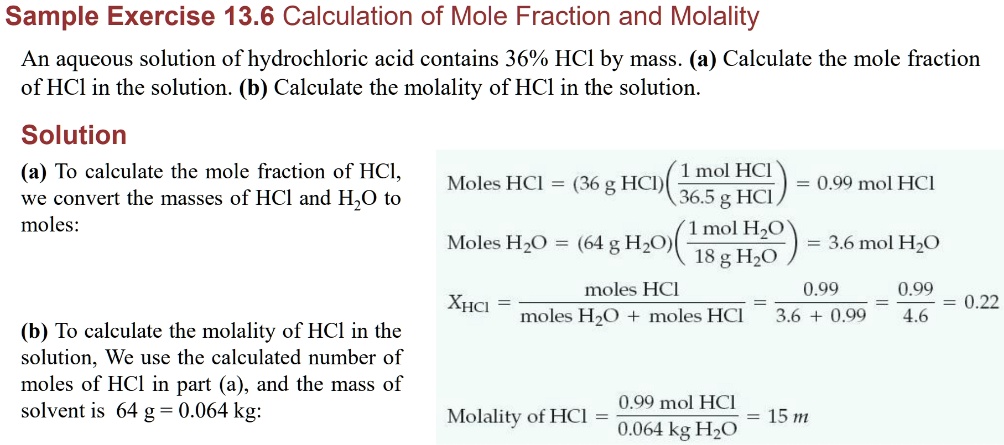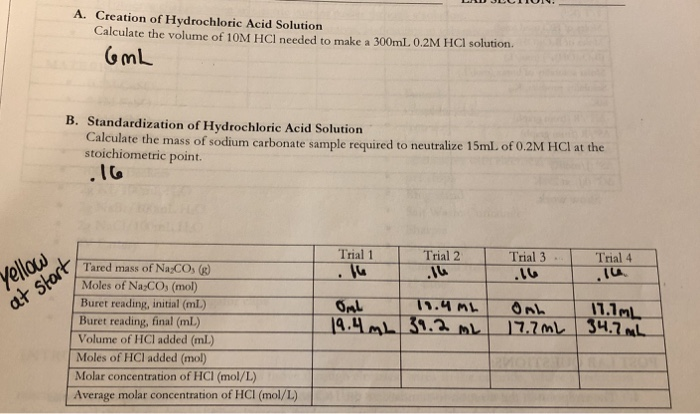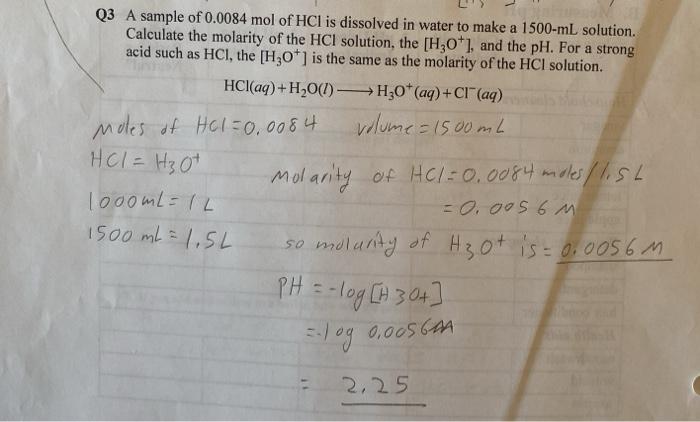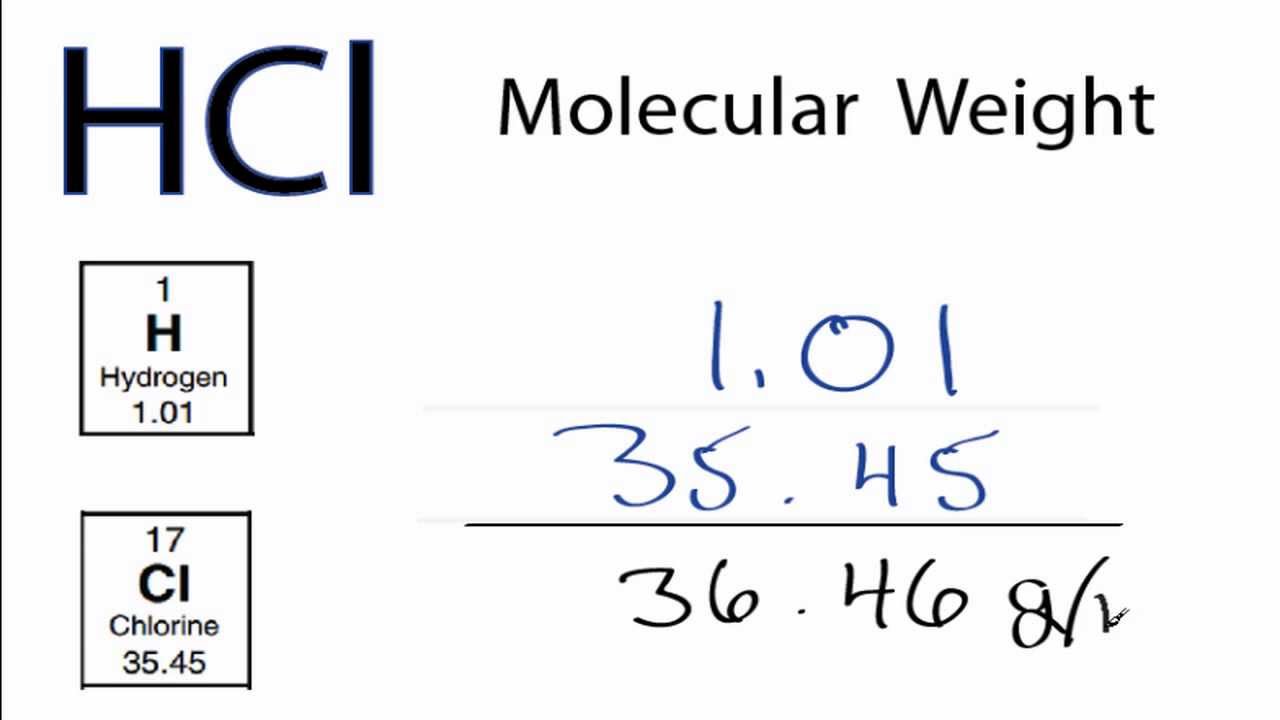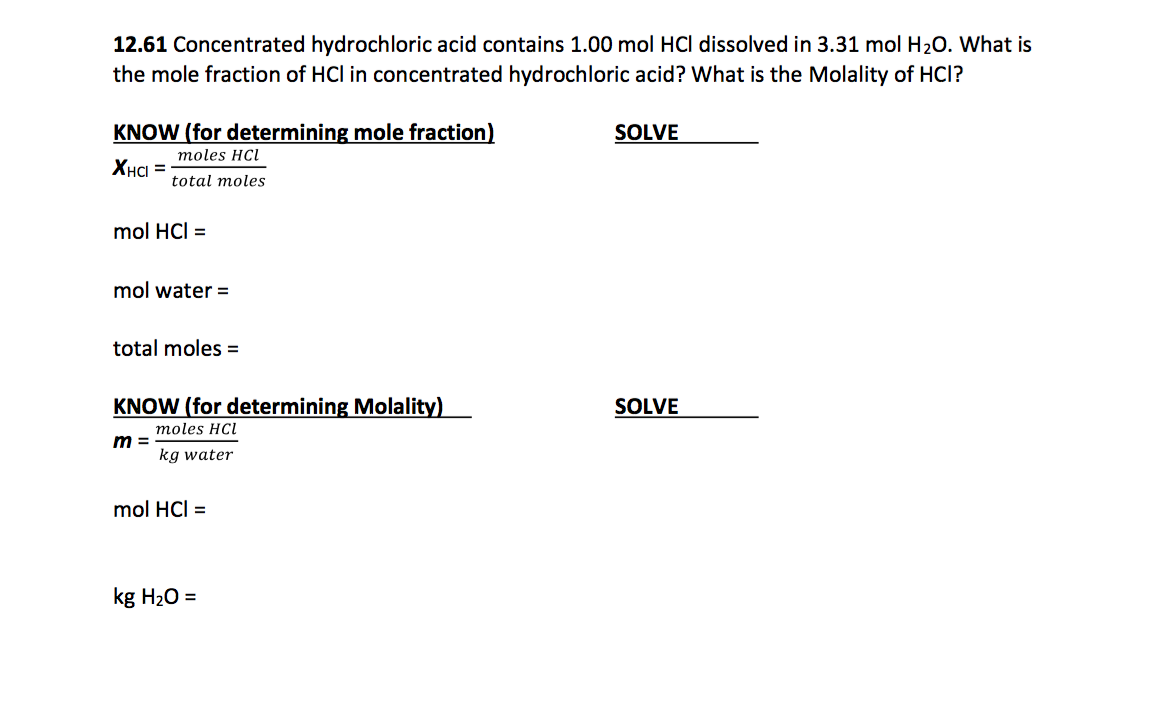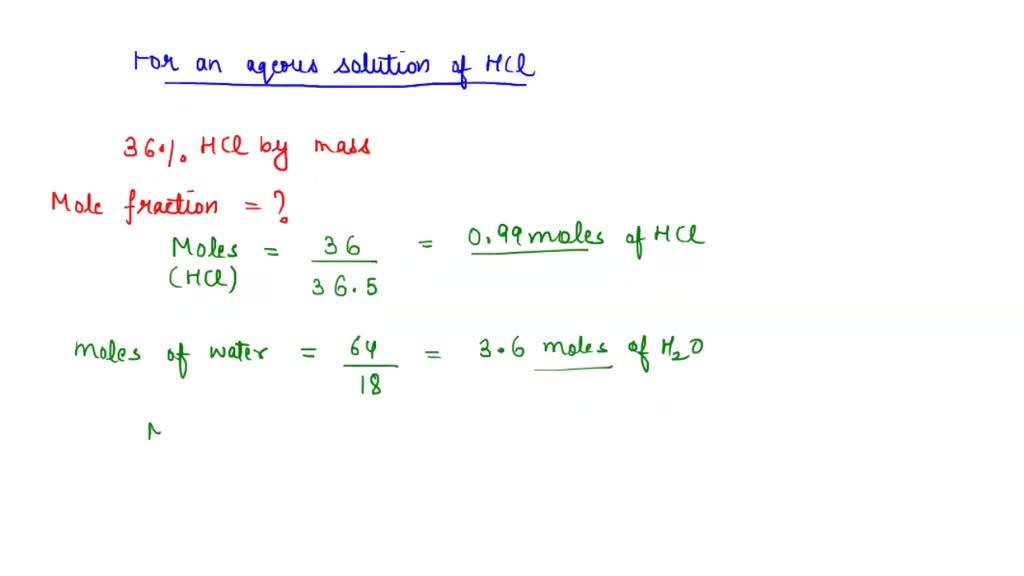
SOLVED: Sample Exercise 13.6 Calculation of Mole Fraction and Molality An aqueous solution of hydrochloric acid contains 36% HCl by mass. (a) Calculate the mole fraction of HCl in the solution. (b)

SOLVED: Which has the most number of moles of HCl? a. 0.04L of a 0.1M HCl b. 0.01L of a 0.5M HCl c. 0.2L of a 0.2M HCl
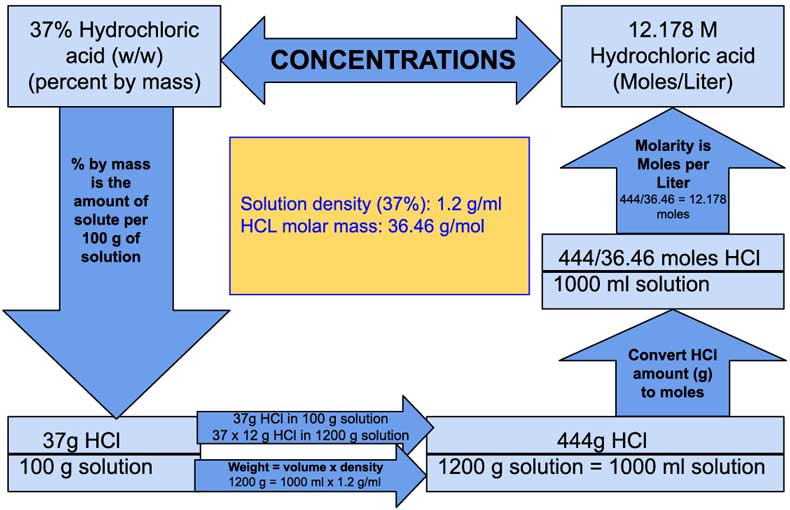
How to Calculate Molarity of 37% (w/w) Hydrochloric acid (HCl), Alternative Method - Laboratory Notes

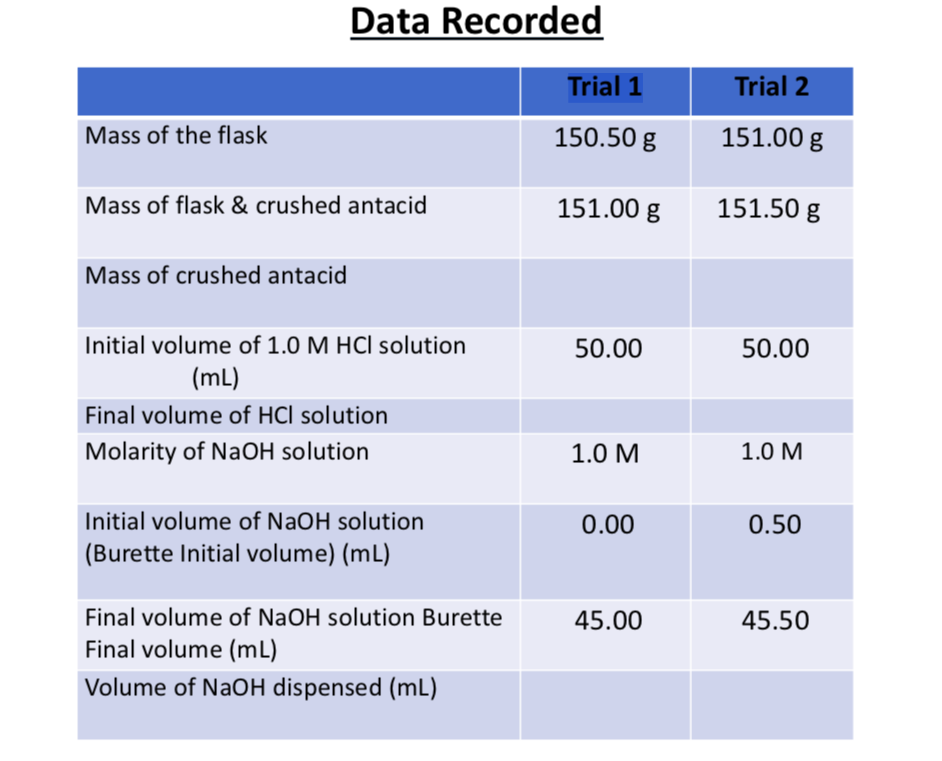
Question Video: Calculating the Concentration of a Hydrochloric Acid Solution Using Experimental Data | Nagwa

If the enthalpy of formation and enthalpy of solution of HCl (g) are-92.3kj /mol and -75.14kJ/mol - YouTube

How many gram moles of HCl will be required to prepare one litre of a buffer solution (containing NaCN and HCN ) of pH 8.5 using 0.10g formula mass of NaCN ?