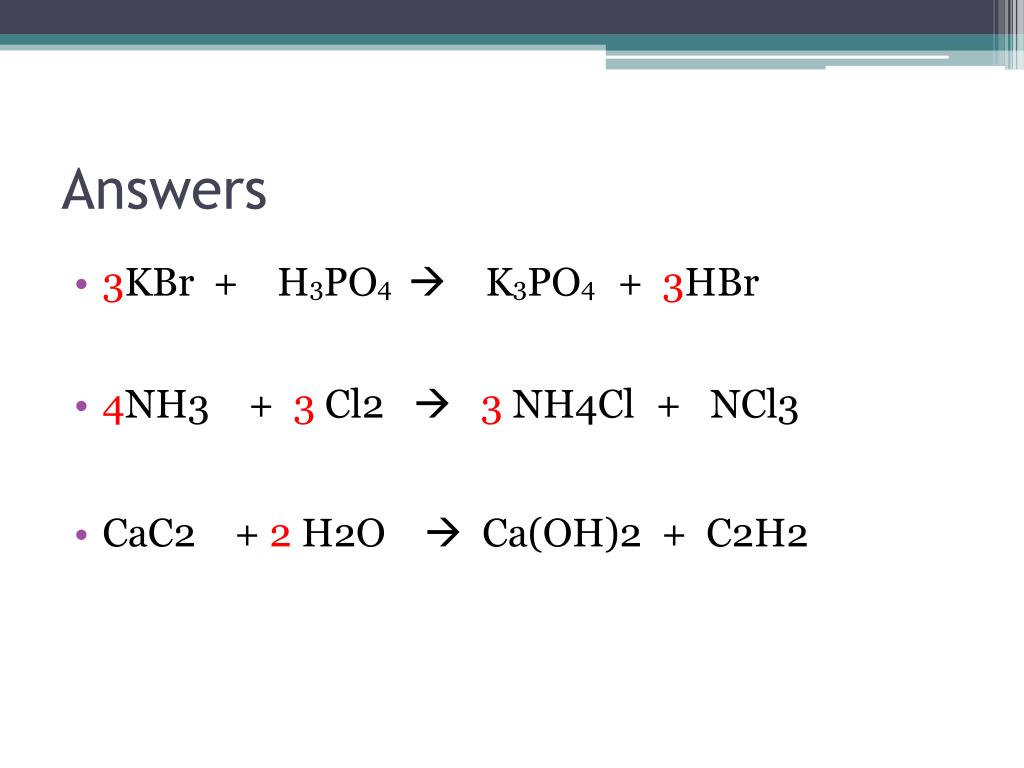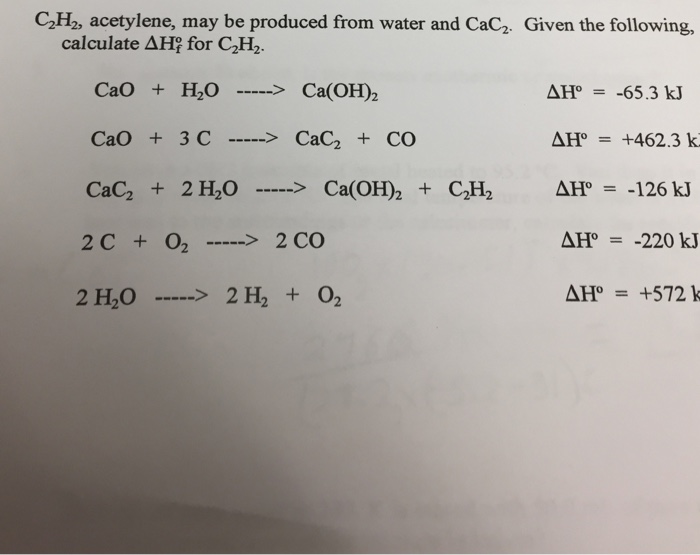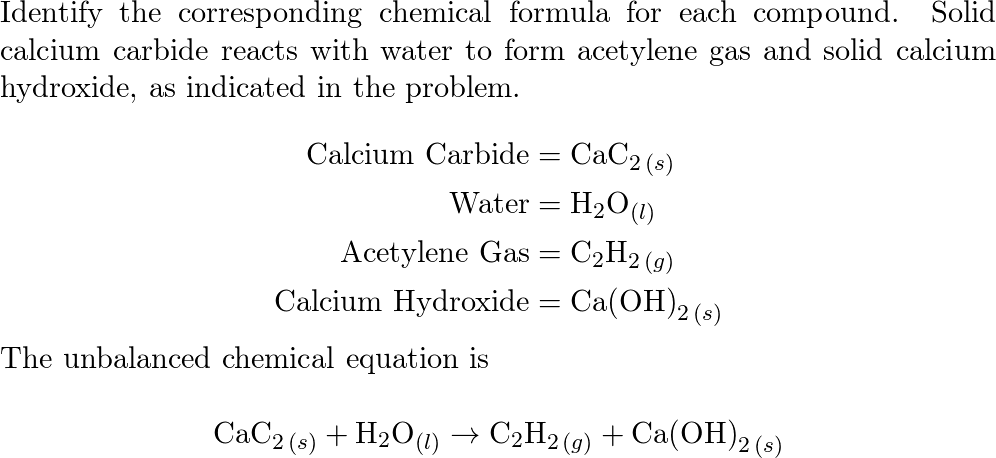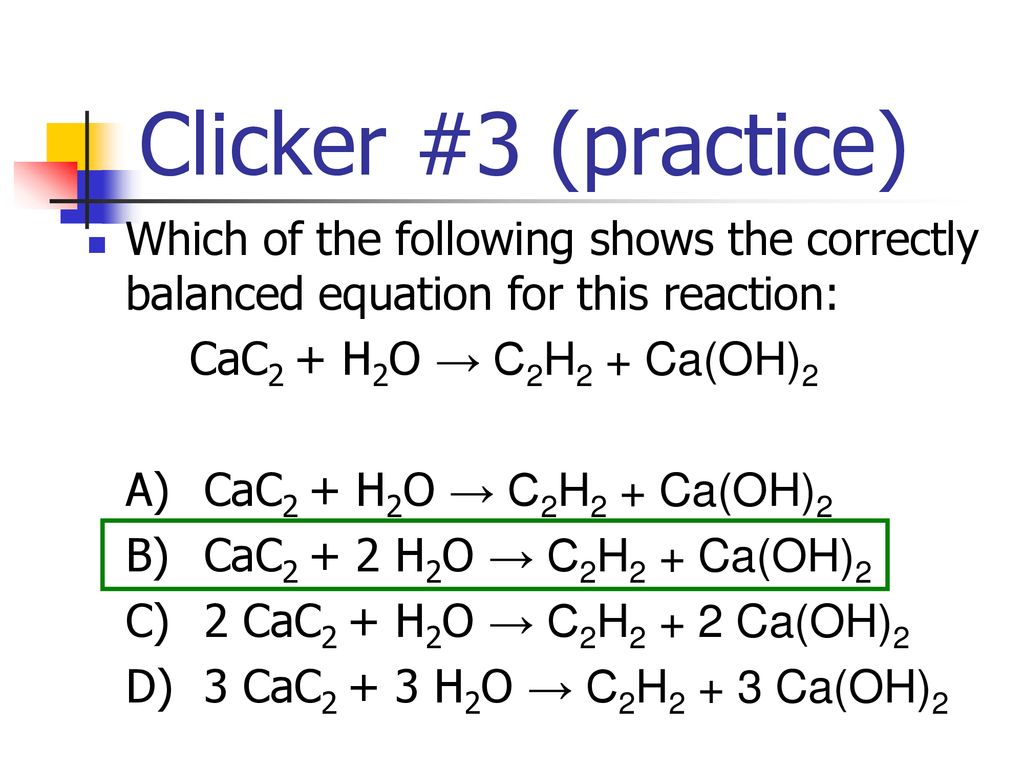
Clicker #1 Which of the following shows the correctly balanced equation for this reaction: H2O2 → H2O + O2 A) 3 H2O2 → 3 H2O + O2 B) H2O2 → 2 H2O + O2. - ppt download

Class11Cac2+H2O=acetylene (calcium carbide react with water)complete reaction explanations in Telugu - YouTube
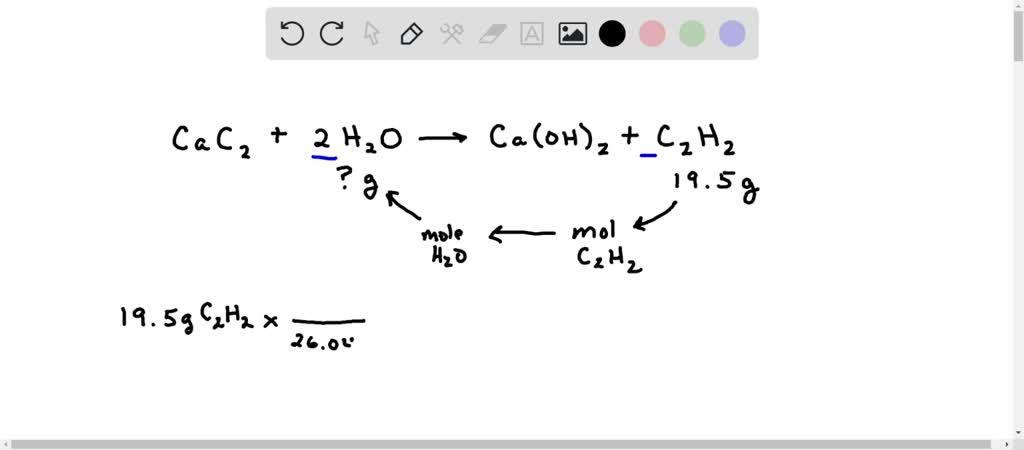
SOLVED: Calcium carbide (CaC2) reacts with water to form acetylene (C2H2): CaC2 (s) + 2 H2O (g) → Ca(OH)2 (s) + C2H2 (g) How many grams of water are required to produce
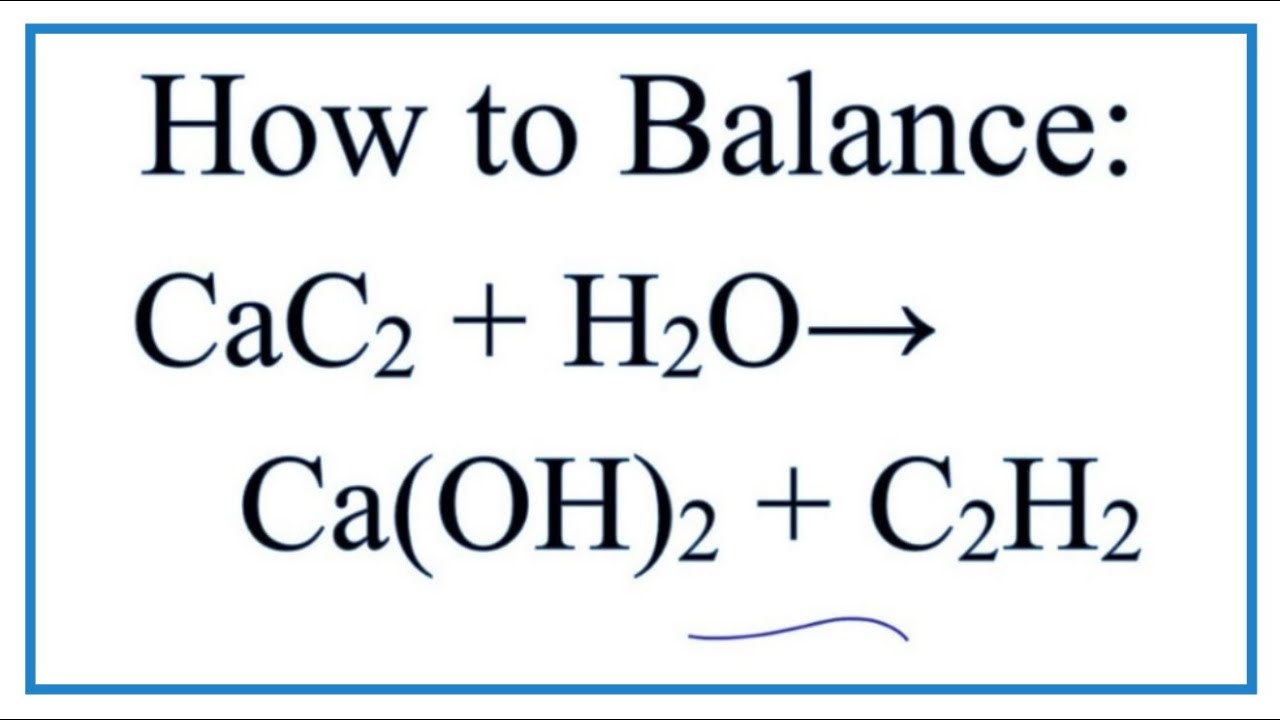
Calcium carbide, CaC2, reacts with water to form ethyne, C2H2, and calcium hydroxide. The equation for the reaction is shown. CaC2(s) + 2H2O(l) → C2H2(g) + Ca(OH) 2(s), which volume of ethyne
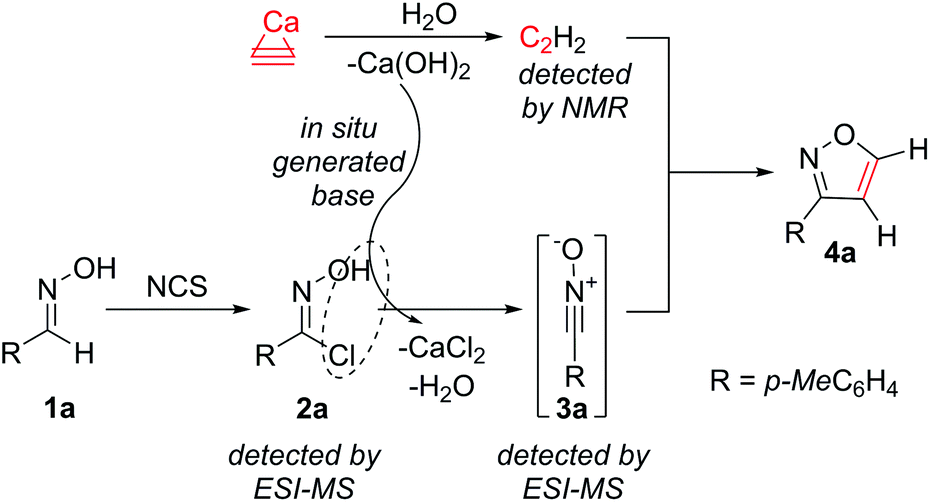
Calcium-mediated one-pot preparation of isoxazoles with deuterium incorporation - Organic Chemistry Frontiers (RSC Publishing) DOI:10.1039/C7QO00705A

Towards C1 chemistry: methanol vinylation by CaC2 in water in the presence of potassium or sodium carbonates - Parshina - 2019 - Journal of Chemical Technology & Biotechnology - Wiley Online Library
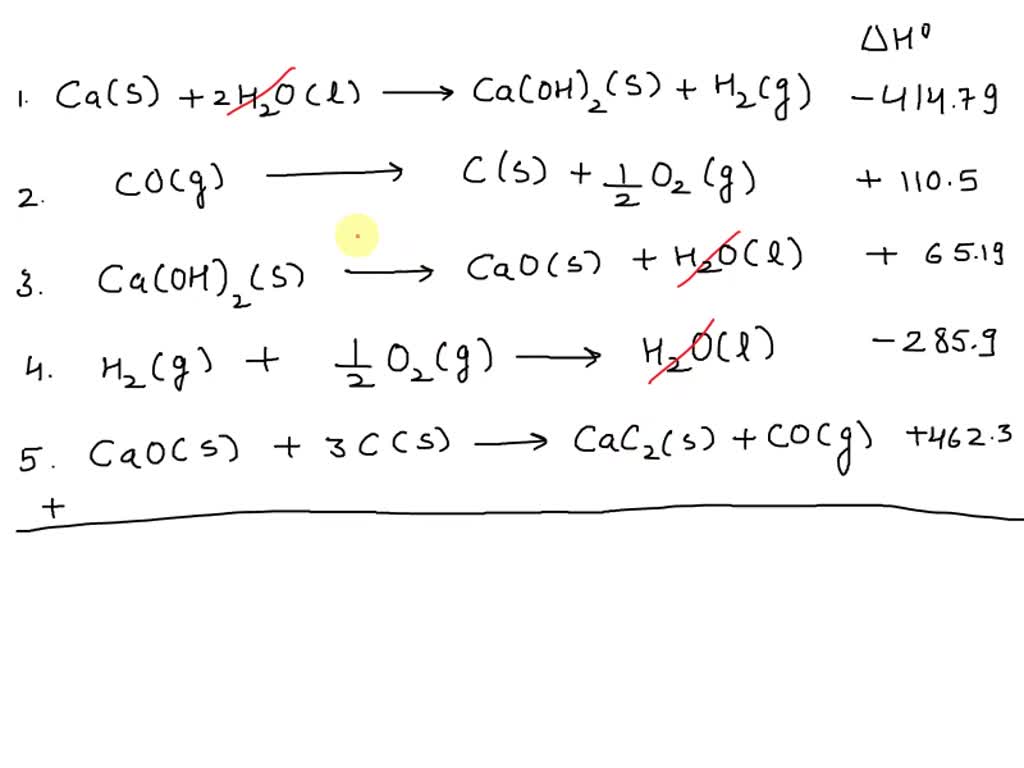
SOLVED: Calculate the standard heat of formation of calcium carbide, CaC2(s), in kJ/mol using the following thermochemical equations. Ca(s) +2H2O(l) 🡪 Ca(OH)2(s) +H2(g) ∆H° = - 414.79 kJ 2C(s) +O2(g) 🡪 2CO(g)
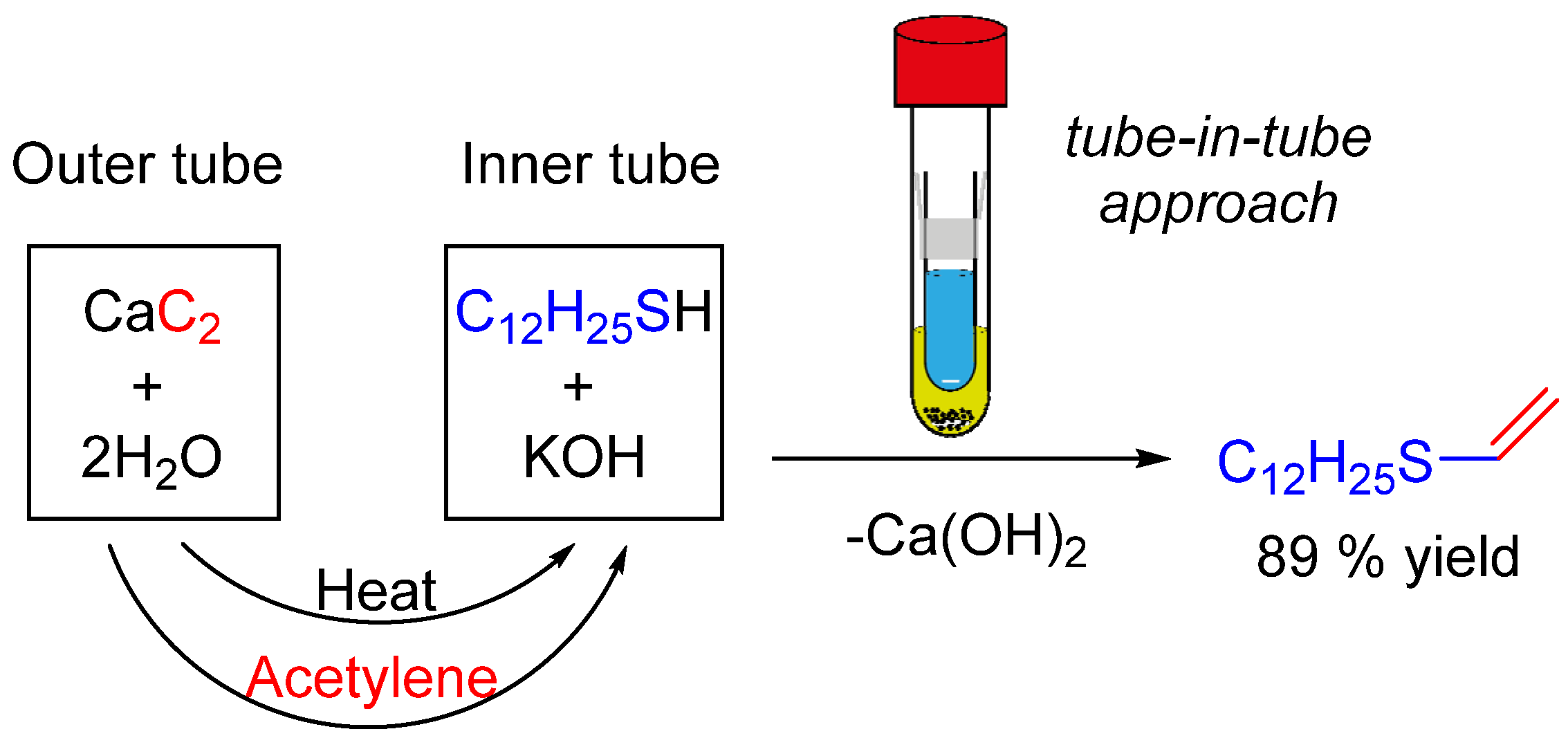
IJMS | Free Full-Text | Thermal Mapping of Self-Promoted Calcium Carbide Reactions for Performing Energy-Economic Processes
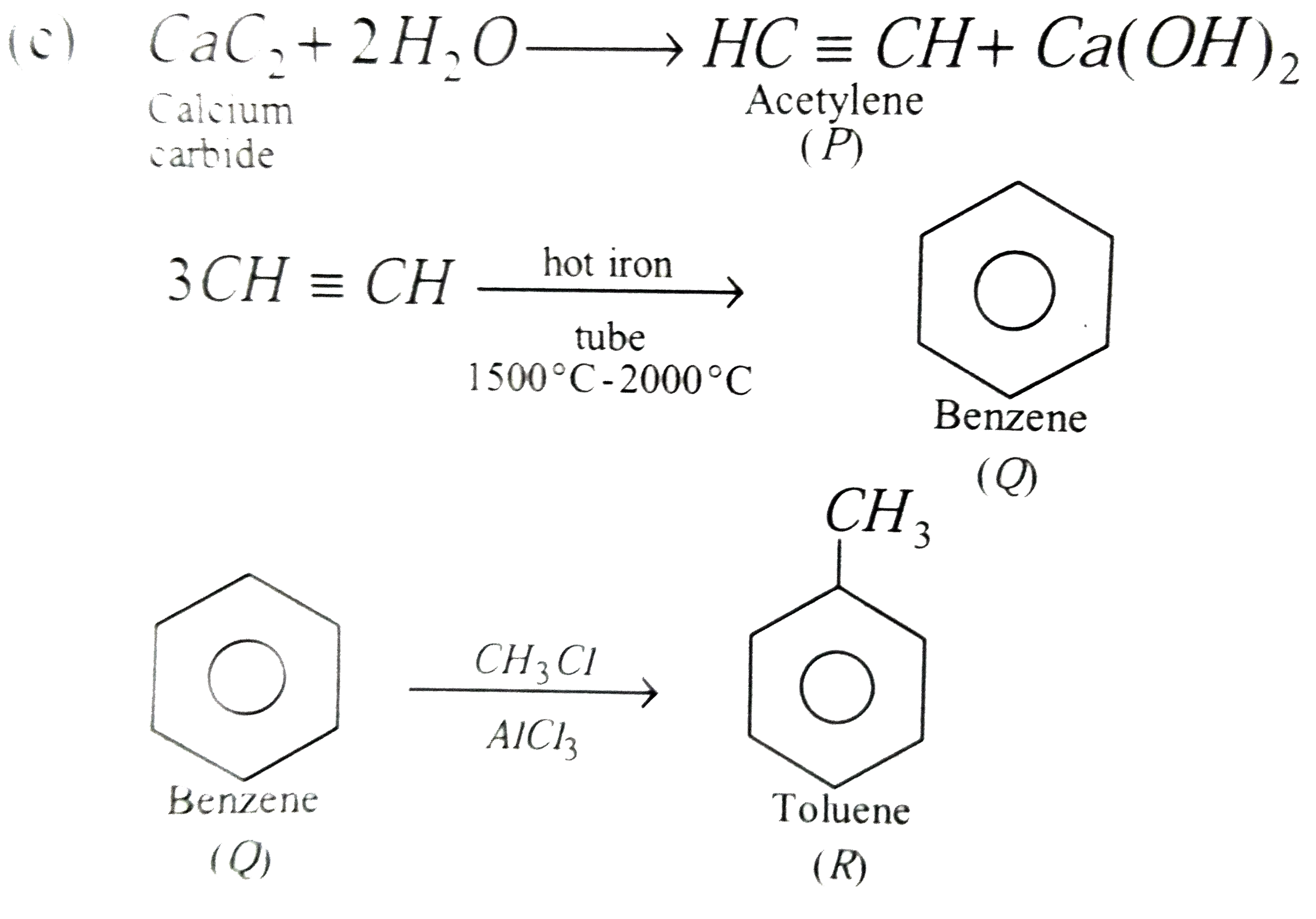
In the following reaction, the product 'R' is: CaC(2) overset(H(2)O)to P overset("hot iron")underset("tube")to Q overset(CH(3)Cl)underset(AlCl(3))toR